The Bond Between Which Two Atoms Is Most Polar
The chemical bond between which two atoms is most polar. This causes the molecule to have a slight electrical dipole moment where one end is slightly positive and the other is slightly negative.

Nonpolar Covalent Bond Covalent Bonding Chemistry Basics Chemistry Lessons
1 point O A covalent bond would form because the electron would be shared so both hydrogens have a full stable shell.

. The molecule with the polar bond that has the greatest difference in electronegativity is the most polar. This is because O is more electronegative than N which is more electronegative than carbon. The bond between which two atoms is most polar 1C-O 2OFF 3он-0.
Which statement explains why a C-O bond is more polar than a F-O bond. According to the video if the difference in electronegativity between two atoms is less than 05 then the bond is considered. The covalent bond is the chemical bond between atoms where electrons are shared forming a molecule.
When electrons are shared between two atoms but there is an uneven of sharing of electrons then the bond between the two atoms is considered. Which bond is most polar Regents. Which formula represents a molecule with the most polar bond.
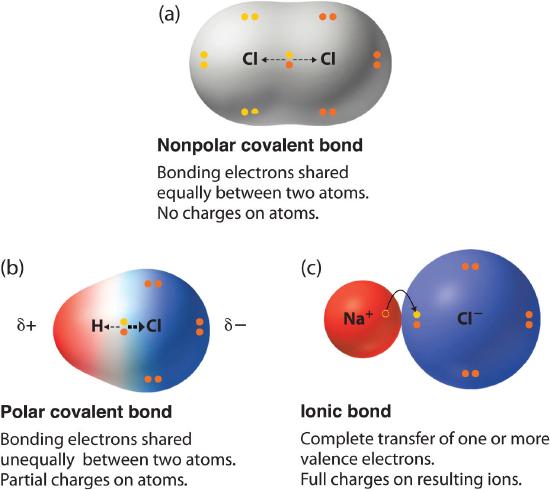
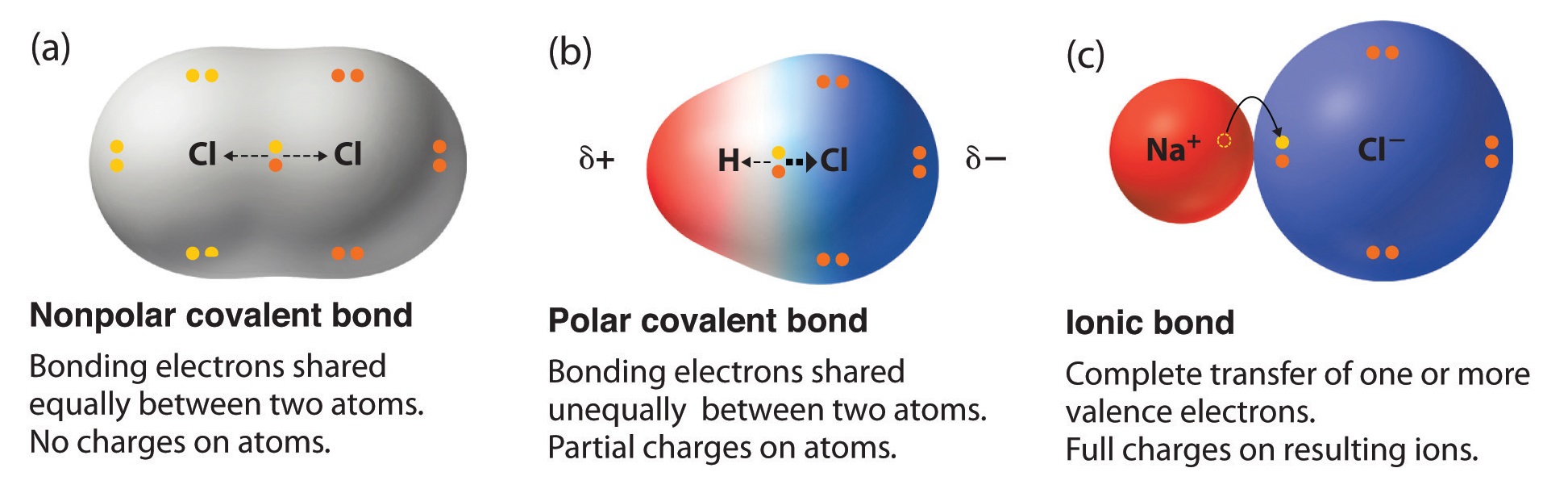
The polarity of a bond is given by the difference in electronegativity between the two atoms that form said bond. The electrons in a bond between two iodine atoms I2 are shared. Equally and the resulting bond is nonpolar.
8 Is an HH bond a hydrogen bond. Bond Polarity and Molecular Polarity PART 1. 12 When 2 hydrogen atoms share electrons What is the result.
The bond between which two atoms has the greatest degree of polarity. The O-H bond has the highest electronegativity difference hence it is he most polar bond. The order of the polarity of the bonds of C N and O with hydrogen is as follows O-H N-H C-H.
63 votes The polar covalent bond or heteropolar covalent bond occurs between atoms of different elements but the two atoms must have an electronegativity difference of less than 19. Arrange these bonded pairs in order of increasing polarity from the least polar to the most polar. O An ionic.
6 What happens when two hydrogens bond. The least polar would be Br-Br because it is the same element therefore there is no element attracting more electrons than the other. 7 Can hydrogen bonds form between two hydrogen molecules.
This is because O is more electronegative than N which is more electronegative than carbon. 13 What kind of bond is formed. Explain in terms of electronegativity why an H-F bond is expected to be more polar than an H-I bond.
For example a carbon-oxygen bond is more polar than an oxygen-fluorine bond because the difference in electronegativity for oxygen and carbon is greater than the difference between fluorine and oxygen. 10 Is methane a double covalent bond. Which kind of bond would form between two hydrogens.
The C-O bond is more polar than the C-N bond which is more polar than the C-C bond. Questions in other subjects. 11 Why can a hydrogen atom form a covalent bond.
To determine the polarity of a covalent bond using numerical means find the difference between the electronegativity of the atoms. If two atoms in a covalent bond are the same 0-0 the attraction for the electrons. A chemical bond that involves the equal sharing of electrons would be an _____ bond.
The electrons in a bond between two iodine atoms are shared. Covalent bonds are established between non-metallic elements such as hydrogen H oxygen O and chlorine Cl. In a polar covalent bond the electrons shared by the atoms spend a greater amount of time on the average closer to the Oxygen nucleus than the Hydrogen nucleusThis is because of the geometry of the molecule and the great electronegativity difference between the Hydrogen atom and the Oxygen atom.
The electrons in a bond between two iodine atoms i2 are shared. A polar bond is a covalent bond between two atoms where the electrons forming the bond are unequally distributed. The quick answer - right from the get-go since nitrogen is one of the most electronegative elements in the periodic table the bond it forms with hydrogen will be the most polar out of all those listed.
The polarity of a bond is given by the difference in electronegativity between the two atoms that form said bond. C-H C-S H-FC-N C-0 and H-Br. The C-O bond is more polar than the C-N bond which is more polar than the C-C bond.
A covalent bond in which the bonding electrons are shared equally between the two atoms. The bond between which two atoms is most polar. BOND POLARITY Each kind of atom has a certain attraction for greediness for the electrons in the bond they share.
The most polar would be H-C because Carbon is. If the result is between 04 and 17 then generally the. 9 Is h2 a double covalent bond.
The ionic bond occurs when the difference in electronegativity between the two elements that intend to bond is greater than 19. The quick answer - right from the get-go since nitrogen is one of the most electronegative elements in the periodic table the bond it forms with hydrogen will be the most polar out of all those listed. Apr 11 2015.
Using your periodic table you can locate each element and see the distance between the two and generally the further apart they are the more polar the bond will be. A covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal. English 09012021 0100 Fourscore and seven years ago our fathers brought forth on this continent a new nation conceived in liberty and dedicated to the proposition that all men are created equal.
The order of the polarity of the bonds of C N and O with hydrogen is as follows O-H N-H C-H. 1 C-N 3 S-Cl 2 H-H 4 Si-O. The answer is b N - H.

Polar And Nonpolar Cl H Electronegativity Is The Ability Of An Atom To Pull Or Attract Electrons Shared Between Two Atoms An Atom Ppt Download

Unixporn Chemistry Writing Inspiration Linux
If The Difference In Electronegativity Between Two Atoms Is Exactly 0 5 Would The Atoms Form A Nonpolar Covalent Bond Or A Polar Covalent Bond Quora

Water Molecules Are Polar In Nature Water Molecules Are Basically H 2o Molecules Which Have Bent Shapes Also O Atom Is Water Molecule Chemistry Molecules

Is Hbr Polar Or Nonpolar Hydrogen Bromide Molecules Polar Ball Exercises

Bond Polarity Flashcards Quizlet

High School Chemistry Core Concept Cheat Sheet 15 Chemical Bonding Key Chemistry Terms Using Teaching Chemistry Chemistry Classroom High School Chemistry
1 9 Electronegativity And Bond Polarity Review Chemistry Libretexts

Bond Polarity Chemistry For Non Majors

Is It An Ionic Covalent Or Polar Covalent Bond Covalent Bonding Bond Ionic

Is Hi Polar Or Non Polar Hydrogen Iodide Chimie Physique Chimie

Lesson Explainer Polar And Nonpolar Solvents Nagwa

Tetryonics 56 02 Sigma Pi Bonds Are The Basic Chemical Bonds That Lead To Complex Compounds Being Created By Elements In Search Of Equilibrium

Polar Bonds And Molecules Notes 16 3 Covalent Bonds Bond In Which Two Atoms Share A Pair Of Electrons 1 Single Bond 1 Covalent Bonding Molecules Writing
The Bond Between Which Two Atoms Has The Greatest Degree Of Polarity In The Following Molecule Socratic
8 7 Bond Polarity And Electronegativity Chi Chemistry Libretexts

Covalent Bonds Chemwiki Covalent Bonding Chemistry Textbook Chemical Bond


Comments
Post a Comment